Background:
A significant subset of Diffuse large B-cell lymphoma (DLBCL) patients (30%-40%) face treatment failure, necessitating effective salvage chemotherapy. We conducted a phase II study evaluating the efficacy and safety of chidamide plus R-GDP (rituximab, gemcitabine, dexamethasone, cisplatin) in relapsed/refractory (r/r) DLBCL patients.
Methods:
This open-label, single-arm study included 27 patients with r/r DLBCL who were ineligible for autologous stem cell transplantation. Prior to the induction monotherapy phase, chidamide was administered orally at a dose of 30 mg twice a week for one week. The combination therapy phase consisted of oral chidamide at a dose of 20 mg twice a week for two weeks, with a one-week discontinuation period, along with intravenous administration of rituximab, gemcitabine, dexamethasone, and cisplatin on a 21-day cycle.
Results:
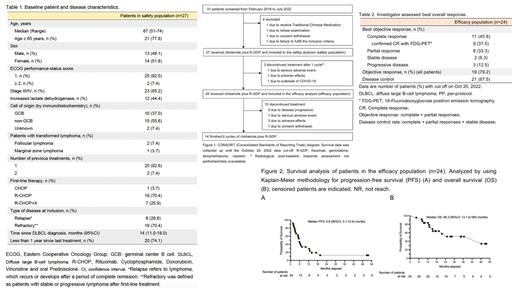
A total of 27 patients received at least one dose of Chidamide plus R-GDP and were evaluated for safety. The median follow-up period was 17.0 months (range: 3.5 to 55 months). Radiological response assessment was available for 24 patients, who constituted the efficacy population. Fourteen patients completed six cycles of chidamide plus R-GDP (per-protocol population), while 10 patients discontinued treatment at the data cutoff. The baseline characteristics of enrolled patients included a median age of 67 years, predominantly older than 65 years. Approximately half of the patients exhibited elevated lactate dehydrogenase levels. All patients had received prior therapy with R-CHOP or equivalent chemoimmunotherapy. The majority of cases were classified as stage III/IV and non-germinal center B-cell-like (non-GCB) type by immunohistochemistry. Refractory or relapsed DLBCL within 1 year since the last treatment was observed in 74.0% of patients.
Efficacy Outcomes:
The investigator-assessed best objective response rate (ORR) was 79.2% (95% CI: 75.1% to 83.3%), with a complete response (CR) rate of 45.8% (95% CI: 41.6% to 49.9%) and a partial response (PR) rate of 33.3% (95% CI: 29.3% to 37.4%). The median time to response was 1.2 months, and the median time to CR or CRu (unconfirmed complete response) was 3.9 months. The best disease control rate was 87.5%. Among the patients who completed six cycles of chidamide plus R-GDP, the best ORR was 100%, with 71.4% achieving CR and 28.6% PR. Kaplan-Meier plots reveal that the median investigator-assessed progression-free survival (PFS) for the efficacy population was 5.9 (95% CI, 3.1 to 12.4) months. The median overall survival was 48.3 (95% CI, 13.1 to not reach (NR)) months.
Safety Outcomes:
A total of 13 patients (48.1%) required treatment interruption, primarily due to adverse events (22.2%) and disease progression (18.5%). Among patients who completed six cycles of chidamide plus R-GDP, 57.1% required a chidamide dose reduction. The most frequent treatment-emergent adverse event was anemia, occurring in all patients. Thrombocytopenia was the most common grade ≥3 adverse event leading to treatment interruption or dose reduction, affecting 48.1% of patients. Non-hematologic events included hypocalcemia (59.3%), hyponatremia (55.6%), and hypokalemia (44.4%). Three patients experienced grade 3 pneumonitis, and one patient had grade 3 skin rash.
Conclusion:
Chidamide plus R-GDP salvage chemotherapy demonstrated promising efficacy (ORR: 79.2%; CR rate: 45.8%) in r/r DLBCL patients. Adverse events were manageable but necessitated monitoring. Larger trials are needed to validate these findings and establish chidamide plus R-GDP as a potential treatment option for this challenging population.
Keywords: relapsed/refractory DLBCL, salvage chemotherapy, chidamide, R-GDP, efficacy, safety
Disclosures
No relevant conflicts of interest to declare.